Spinal cord stimulation was approved by the food and drug administration fda in 1989 to relieve pain from nerve damage in the trunk arms or legs and now accounts for about 90 percent of all neuromodulation treatments.
Electrical spinal cord stimulation treatment.
This process goes by many different names including brain machine interface brain controlled interface neural control interface and mind machine interface.
When other pain treatments have failed spinal cord stimulation may be an option.
Spinal cord stimulation was first used to treat pain in 1967.
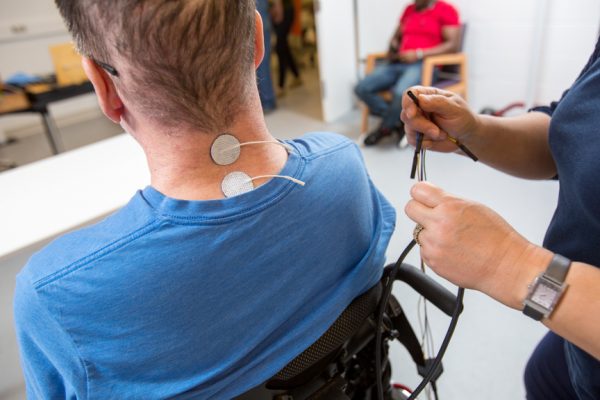
Functional electrical stimulation fes is a treatment method in which low level electrical impulses are applied to nerves or muscles to improve or restore muscle function in people with spinal cord injuries.
What you need to know spinal cord stimulation is used most often after nonsurgical pain treatment options have failed to provide sufficient relief.
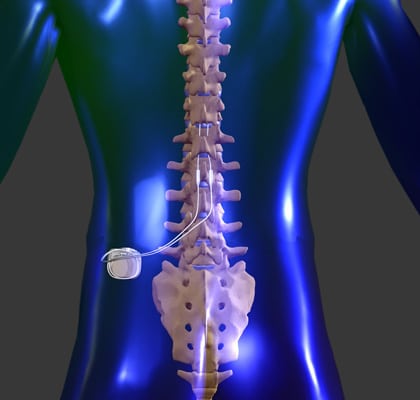
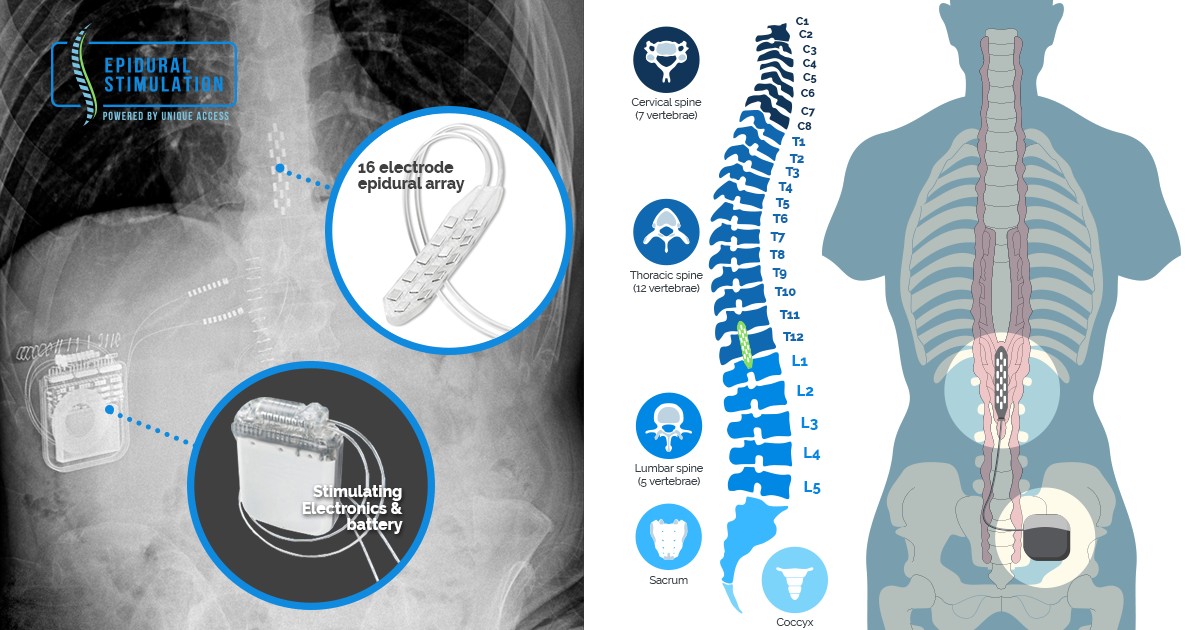
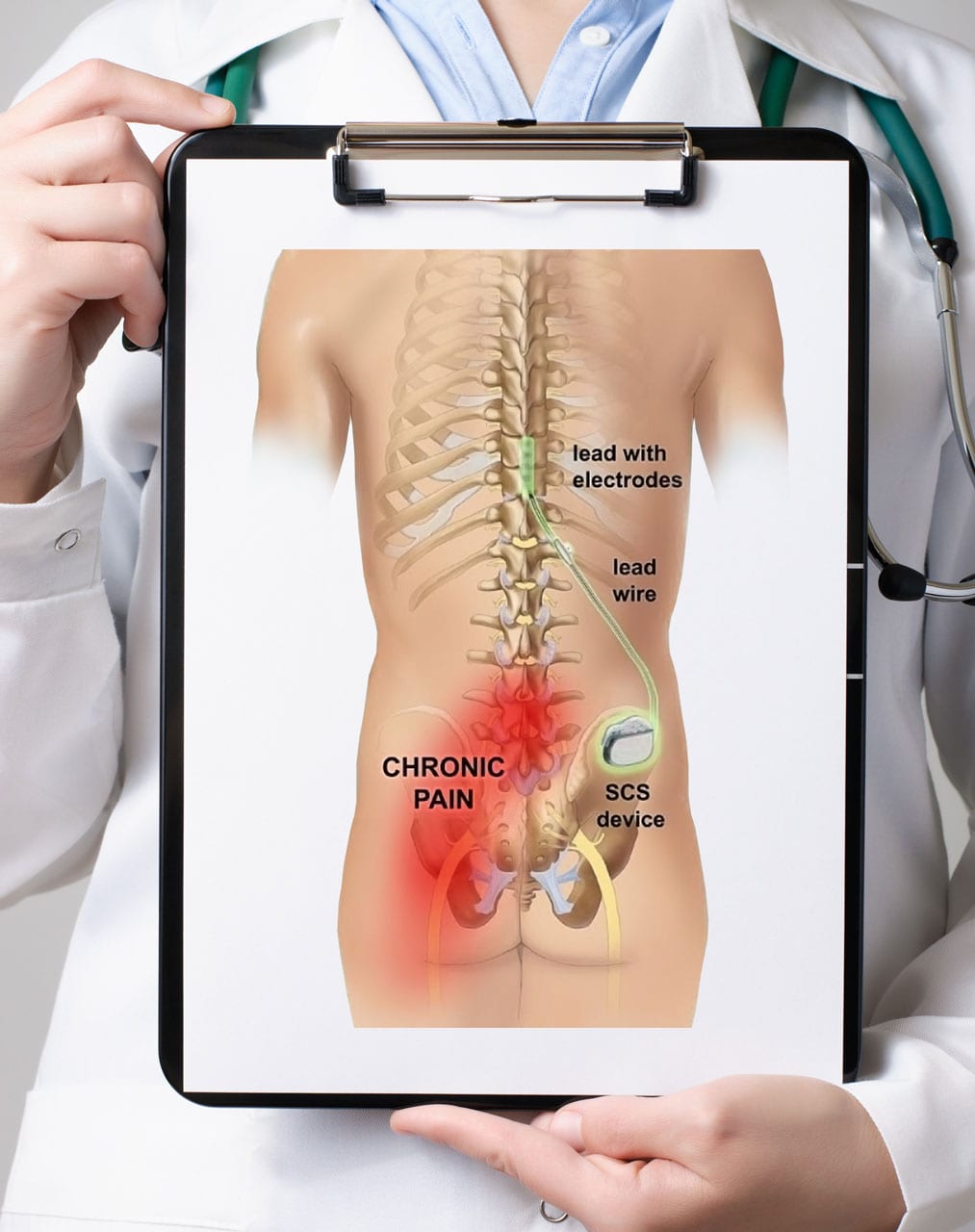
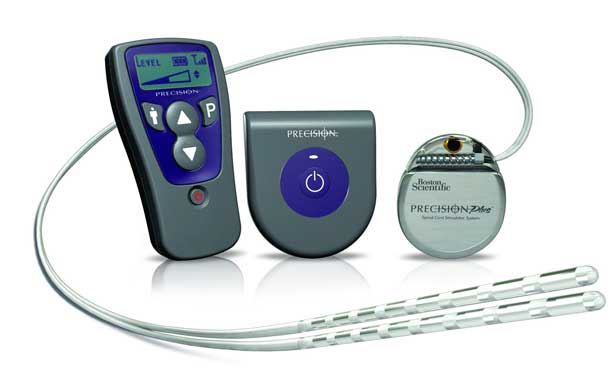
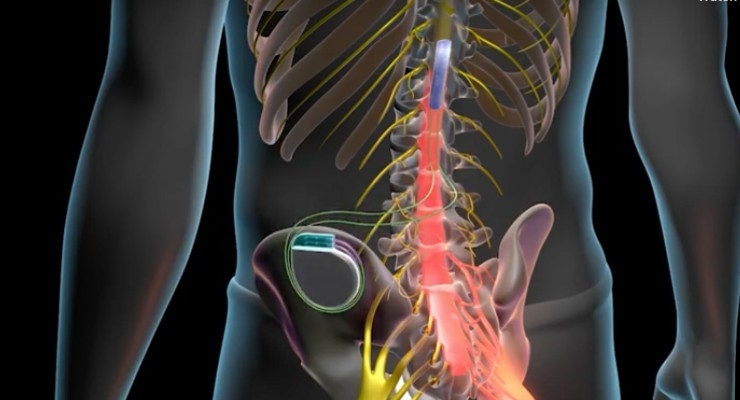
A spinal cord stimulator is an implanted device that sends low levels of electricity directly into the spinal cord to relieve pain.
After a patient has been evaluated and non surgical treatments have been used spinal cord stimulation is considered to help manage chronic pain.
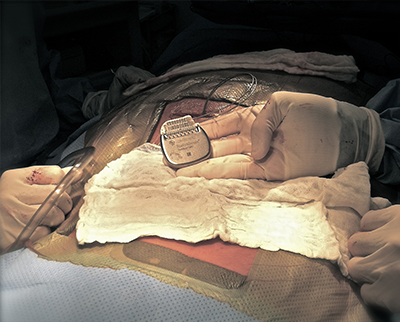
Description a trial electrode will be put in first to see if it helps your pain.
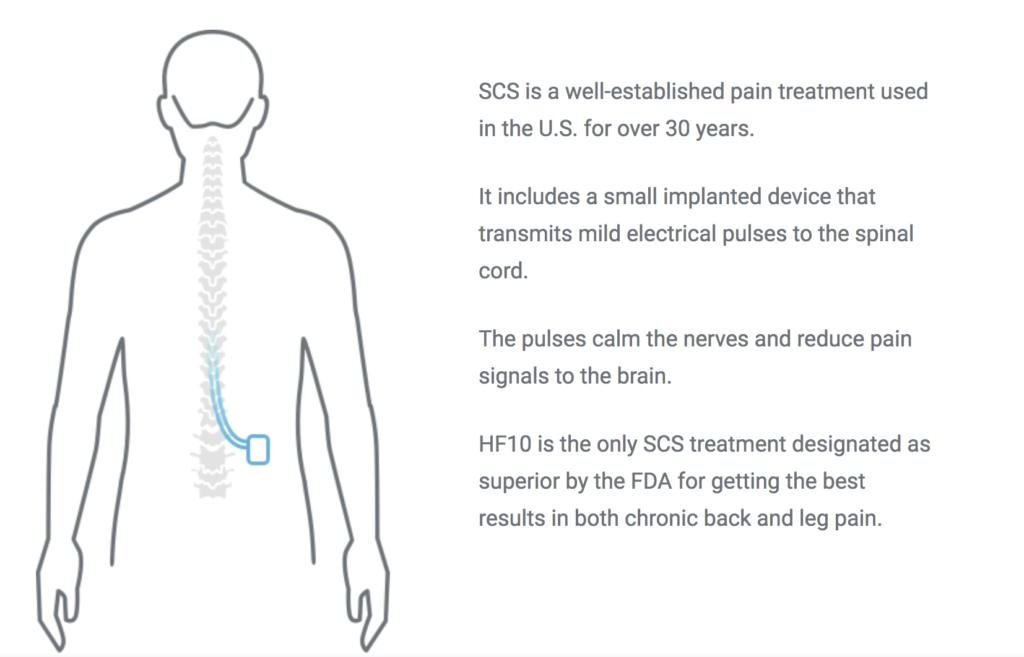
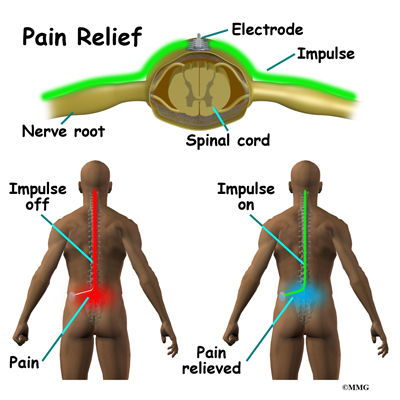
Spinal cord stimulation is a treatment for pain that uses a mild electric current to block nerve impulses in the spine.
Spinal cord stimulation is a procedure that delivers low level electrical signals to the spinal cord or to.
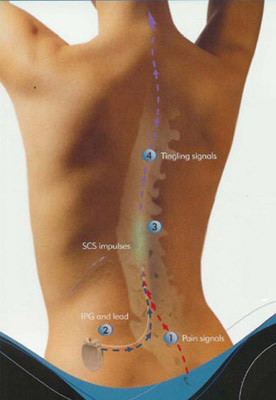
By placing a stimulating electrode over the spinal cord the pain signal cannot be sent up from the spine to the brain.
Spinal cord stimulation for chronic back pain uses electrical pulses to stimulate nerves in the spinal cord with the goal of interfering with the path of pain signals as they travel to the brain.
The first step in the process is a trial period of spinal cord stimulation.